Participants in youth livestock competitions learn more than just how to raise and show animals—they also play a role in our food system. It’s important to know which products and medications are allowed for use on livestock because many of these animals enter the human food chain after the show. By understanding and following the rules, youth help ensure that meat and animal products meet strict safety standards. This not only protects public health but also upholds the fairness of the competition, teaches ethics, and shows responsibility in animal care. Learning these guidelines is key to building a safer and more sustainable future for agriculture.
Youth who raise livestock projects have the responsibility to make sure their animals’ products are safe and healthy. Exhibitors must follow the same rules and regulations as all livestock producers, even for breeding animals that aren’t being sold. To prevent drug residues from entering the human food chain, only FDA-approved products (like medications or treatments) can be used. When using any product—whether it’s over-the-counter or prescribed by a vet—you must follow the label carefully. The label explains which illnesses can be treated, which species can be treated, how to give the medicine, and how long the withdrawal time is.
This guide is here to help you understand the products you can use and how to use them safely. If you’re ever unsure about treating your animal or whether a product is allowed, ask your veterinarian for help.
Jump To:
- What is the FDA’s definition of a drug?
- What is an FDA approved animal drug?
- Can I still show if I give my animal medication?
- How can I tell if a product I intend to give to my animal is allowed?
- Can I use an FDA approved product in a manner that is different from its label?
- Can I use fly spray or other external pest control products?
- Can I use supplements, topicals, and other non-FDA animal health products?
- Key points
- Glossary
- References
What is the FDA’s definition of a drug?
According to the Food and Drug Administration (FDA), there are multiple definitions for a “drug.” For the simplicity of this publication, a drug is going to be referred to as:
- A substance that is recognized by an official pharmaceutical or formulary.
- A substance that is used for the diagnosis, cure, mitigation, treatment, or prevention of a disease or illness.
- Non-food substances that affect the structure or function of an animal’s body. (Food and Drug Administration).
What is an FDA approved animal drug?
All animal medications must go through a detailed approval process with the FDA. When a pharmaceutical company creates a new drug, they submit information to the FDA for review. If the drug meets the FDA's safety and effectiveness standards, it gets approved. An FDA-approved drug means it’s safe and works well when used according to the label. Even after approval, the FDA monitors these drugs by testing batches for quality and checking labels for accuracy.
The FDA also sets a withdrawal time for each drug to make sure no drug residues accidentally enter the food supply. The dose, duration, and route of administration must all be followed exactly for the withdrawal time to be valid. If any of these factors change—like giving more medication, extending the treatment, or using a different method—the withdrawal time no longer applies. This would be considered extra-label use, which is illegal for producers to do without a veterinarian's guidance.
Some FDA-approved products are available over-the-counter and don’t need a veterinary prescription. Others are restricted and require a prescription or a veterinary feed directive (like a prescription, but for products that are fed to the animal in their feed). Through this thorough process, the FDA helps keep the public and animals safe.
Can I still show if I give my animal medication?
The rules depend on the type of medication used to treat the animal. Medications that cause sedation or enhance performance—even if it’s just a side effect of an otherwise necessary treatment—may not be allowed. Using these could disqualify the animal from being shown. However, if the medication doesn’t cause sedation or enhance performance, the animal can likely still be shown, depending on the show’s rules.
For animals being auctioned, the rules are a bit different. If the medication has a withdrawal time that hasn’t passed by the auction date, the animal may not be allowed to be sold. In some cases, the animal might need to be held back until the withdrawal time has passed and then harvested safely at a later time.
But remember, if an animal is sick or injured, it must be treated—even if it means disqualification from the show or sale. When you care for an animal, you’re responsible for treating it ethically. Not treating a sick animal could be considered animal abuse. Always check the show rules for medication guidelines, and talk to your vet if unsure. They might be able to suggest an alternative product.
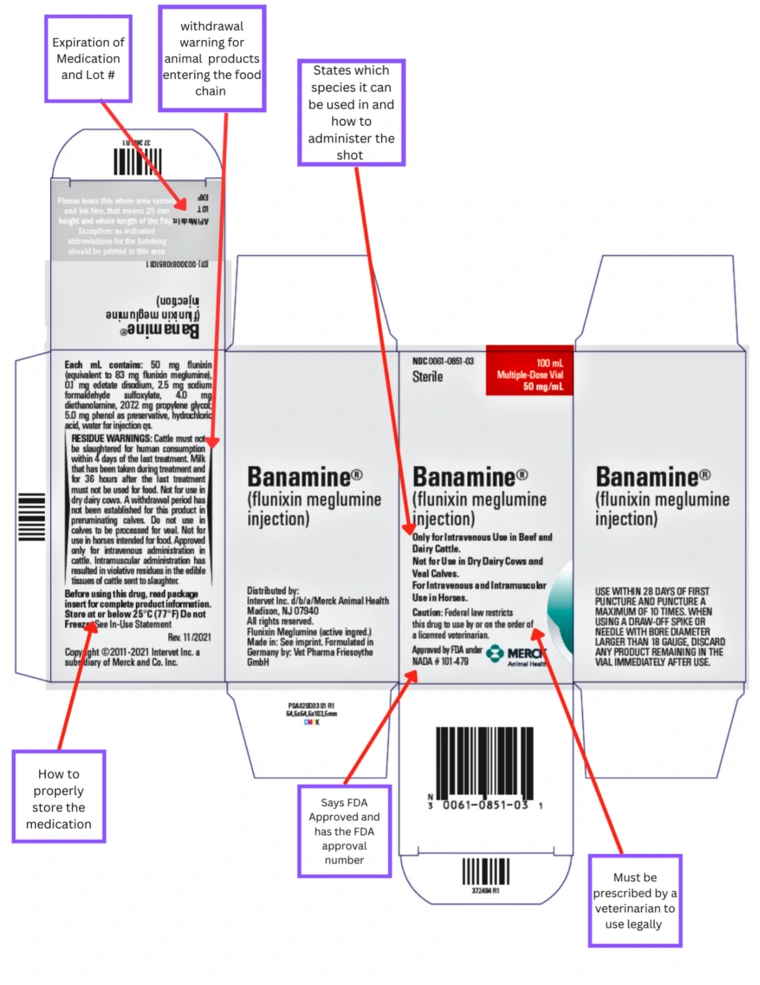
How can I tell if a product I intend to give to my animal is allowed?
When raising animals intended for food production (meat, milk, eggs, etc.), it is crucial that you examine the labels of all health products that are going to be given to your livestock project. As stated previously, the FDA regulates all animal health products. You, as the animal producer, are legally obligated to follow the instructions provided on the label. This includes: the animal species, reason for treatment, dosage, route of administration, and withdrawal time. Products that are not labeled specifically for use in food producing animals may not be used. The products labels will include the following information:
- A New Animal Drug Application (NADA) or Abbreviated New Animal Drug Application (ANADA) number, along with the statement “Approved by FDA under NADA # XXX-XXX” or “Approved by FDA under ANADA # XXX-XXX”.
- A statement on species/class of species and route(s) of administration allowed/not allowed.
- Caution: This may include a recommendation to consult with your veterinarian for assistance, or restrict the drug to use by or on the order of a licensed veterinarian (prescription only).
- Description: The amount of active and inactive ingredients in each milliliter of the product
- Pharmacology: Information on how the product works in each listed species
- Indications: the diseases or conditions the drug is approved to treat in each allowed species or class of species (i.e., dry vs lactating dairy cattle, beef cattle, veal calves, laying hens)
- Dosage and Administration: The amount (dosage) of medication to give and how to give it (route of administration) information for each species/ condition listed on the label.
- Contraindications: What conditions the product should NOT be used under
- Residue Warnings: how long you must wait after the last treatment is given before an animal is harvested for human consumption, or the milk or eggs may be consumed.
- Precautions: Specific warnings that the drug may have an effect on animals with specific conditions (such as pregnancy), known or potential adverse effects if given with other medications, etc.
- Safety: information on potential overdose toxicity or other concerns
- Adverse reactions: possible adverse reactions that were noted during the drug testing and approval process
- How supplied: how this medication is available (i.e. a paste, a liquid, a tablet)
- Storage information: recommended storage temperatures and conditions to ensure the product remains effective
- References: The studies used to determine the above information
Can I use an FDA approved product in a manner that is different from its label?
Using an FDA approved product in a manner that is inconsistent with its label is called extra- label or off-label use. This includes altering the dosage amount, route of administration, or using the product to treat an illness other than what is stated on the label. In 1994, congress passed the Animal Medical Drug Use Clarification Act, which gave licensed veterinarians the ability to prescribe both approved human and animal drugs for extra-label use. Using a product in this way requires the supervision of a veterinarian and a written prescription (even for over-the-counter products) because it can change the withdrawal time. There are restrictions on which drugs may be prescribed for extra-label use, and you will need to have a valid veterinary-client-patient relationship. If you have any questions about the drug(s) that have been prescribed for your animal or how to use an over-the-counter product, contact your veterinarian.
Can I use fly spray or other external pest control products?
Much like how the FDA regulates drugs, the Environmental Protection Agency (EPA) regulates topical pest products that are intended to remain on the skin and treat external parasites (About Pesticide Registration 2024). This means that fly sprays and other external parasite treatments will not have a NADA or ANADA number. However, they will have an EPA registration number and an EPA establishment number on their label. On the label, it will have similar information regarding species/ class of animals the product may be used on, the pests it is intended to treat, the application rates and methods, safety information, and withdrawal times if applicable. As with other drugs or animal health products, following all labeling instructions and checking with your veterinarian if you have any questions is important.
Can I use supplements, topicals, and other non-FDA animal health products?
While some supplements, topical products, and animal health products are relatively benign, others may contain ingredients that are not allowed for food producing animals. This is especially important for products targeted for horses or pets, where drug residues entering the human food chain aren’t a concern. Since these products are not under the jurisdiction of the FDA, there is no oversight or mandatory testing to ensure they are free from illegal substances. These products can be easily spotted as they lack a NADA or ANADA number on their packaging or label. However, it’s also common to see people using over-the-counter human medications, like Tinactin™, to treat conditions such as ringworm. In these cases, a withdrawal time must be established by FARAD if a vet approves the medication for use. Most veterinarians will recommend an animal-approved medication (such as ketoconazole or ANIMAX™) before suggesting or prescribing a human medication.
Many supplements and calming agents contain ingredients that have not been approved by the FDA. For example, Calf Calm™ has valerian root as one of its ingredients, which has not been FDA approved. Products that contain caffeine, nicotine, melatonin, CBD, and many other human safe products are also not allowed. Sticking to FDA/ EPA approved products and following their labels is the best way to ensure you are following all legal guidelines for raising food animals.
While there is no exhaustive list of unapproved products in food animals, the following list represents the most common illegal medications, supplements, and products used and found in livestock projects:
- Human medications such as Tylenol™, Advil™, Pepto Bismol™, etc.
- Human topical medications such as pain gels and lotions
- Caffeine (energy drinks, coffee, chocolate, etc.)
- Nicotine
- CBD
- Melatonin
- Illegal drugs such as marijuana, cocaine, etc
- Tranquilizers and sedatives (acepromazine, valerian root, etc.)
FDA approved products for horses/companion animals that are not allowed for food animals (nitrofurazone, clenbuterol, phenylbutazone, etc.)
Additionally, the following actions constitute illegal drug use and may violate exhibition rules:
- FDA approved over the counter product that was used inconsistently with its label and without a veterinary prescription or oversight.
- FDA approved prescription only products that were used without veterinary oversight.
- FDA approved product given to an animal that will be marketed before the withdrawal period.
- FDA approved product given to an animal that violates specific show rules (pain relievers within 5 days, etc.).
It is the livestock exhibitor’s responsibility to act in an ethical, responsible manner toward their livestock projects. If an animal is sick or injured, they must be treated promptly and quickly, even if this means being disqualified from the show. There are many products out there that are targeted for show animals but are illegal to use on livestock animals, even if your animal is not destined for auction/harvest. Medications and supplements that are used need to be FDA approved and in accordance with their label. If there are any questions about what can and cannot be used, reference the FDA website and ask your veterinarian. Exhibitors are ambassadors for the livestock industry, and they need to make sure that each animal is well cared for, they are producing safe and wholesome products, and ensuring the safety of the food chain.
Key points
- Only use FDA-approved products (like medications, creams, or supplements) on your livestock projects.
- Follow all label instructions and vet prescriptions carefully and keep track of the withdrawal time (how long after the last dose of medication before it's safe to show or sell your animal).
- You can use over-the-counter products as long as you follow the label instructions. If you need to use them differently than the label says, you’ll need a vet's prescription.
- Only vets can give you prescriptions, and you can’t reuse withdrawal times from a previous treatment, even if it was for a similar issue. Withdrawal times can change, especially if the medication was used in an off-label way, so always follow your vet’s current guidance.
- It’s your job to care for your animal if it gets sick or injured, even if it means you might be disqualified from a show, fair, or auction. Never avoid treating a sick or injured animal just because of fair rules or withdrawal times—that could be considered animal abuse.
- Check your fair or show rulebook for any special rules about which products you can use to keep things fair for everyone.
- Work with a veterinarian to build a good relationship so they can help you keep your animals healthy
Glossary
- Food and Drug Administration (FDA): An organization in the United States that is responsible for protecting the health and safety of people and animals. The FDA assesses the safety and effectiveness of human and veterinary medications, biological products, medical devices, consumable foods, cosmetics, tobacco products, and products that emit radiation. These assessments are then used to provide accurate information to the public. The FDA determines which medications and animal health products are allowed to be used in food producing animals, and labels those products appropriately to ensure residues from these drugs do not enter the food chain when an animal is harvested for meat, milk, eggs, etc. (Food and Drug Administration).
- Environmental Protection Agency (EPA): An organization in the United States that is responsible for protecting the environment and human health. Part of this role is to assess the safety and effectiveness of pesticides, including those used on livestock, and determine which products can be used and how.
- Route of administration: How a product should be administered to an animal. This can include orally (by mouth), topically (on top of the skin, such as a pour-on wormer), or injected subcutaneously (under the skin, between the skin and muscle), intramuscularly (directly into the muscle), or intravenously (directly into a blood vein). Some products may allow more than one route of administration, or the route may change based on the type of animal being treated.
- Dosage: The amount of a product to administer to an animal to be effective. This may differ by animal species, class (calves vs cows), or be based on the weight of the animal.
- Withdrawal time: The amount of time after a medication or product is given that it is acceptable to market the animal. This time has been established by the FDA and will vary between products. It may also differ by species, route of administration of the product, and animal product to be consumed (meat vs milk, for example). Withdrawal times will be clearly stated on the label or will be provided by your veterinarian in the event of extra-label product use.
- Extra-Label or Off-Label use: Using a product in a manner inconsistent with its label in any way. This includes a different species or class of animal, at a different dosage, for a disease not listed, or using a different route of administration. Using a product in this way requires a written prescription from a veterinarian, even if the product is available over the counter.
- Veterinary-client-patient relationship (VCPR): Defined by Arizona statute (Arizona Statute § 32-2201), a VCPR requires that a licensed veterinarian assumes responsibility for making medical judgements about the animal(s), that they have knowledge of those animals, that the owner has agreed to follow the veterinarian’s instructions, and they are available for follow up evaluations.
- Violative drug residue: Detectable levels of any drug found in the meat or edible tissue (including milk, eggs, etc.) that exceeds tolerance levels set by the FDA.
- Prescription drugs: Products that require written instructions from a veterinarian (a prescription) to be purchased and used by a livestock owner. A prescription is a set of written instructions to treat an animal or group of animals from a veterinarian with an established VCPR. The prescription will indicate which animals are to be treated, why they are being treated, what product should be used, and the dosage/ instructions for treatment as well as the appropriate withdrawal times for that medication.
- Over the counter drugs: Products that are available to purchase and use by livestock owners without a prescription from a veterinarian, as long as they are used according to the labeled instructions.

References
Antaya, A.M., Dalke, A., Mayer, B., Noelle, S., Beard, J., Blum, American Veterinary Medical Association. (n.d.). The veterinarian-client-patient relationship (VCPR). https://www.avma.org/resources-tools/pet-owners/petcare/veterinarian-client-patient-relationship-vcpr
Beef Quality Assurance. (2020). Antibiotic Stewardship for Beef Producers. www.bqa.org/Media/BQA/Docs/bqa-antibiotics-2020.pdf
Environmental Protection Agency. (2024, January 4). About Pesticide Registration. EPA.gov. https://www.epa.gov/pesticide-registration/about-pesticide-registration
FDA.gov. (2024, January 1). Animal Drugs @ FDA explained. U.S. Food and Drug Administration. https://www.fda.gov/animal-veterinary/approved-animal-drug-products-green-book/animal-drugs-fda-explained
FDA.gov (2021, July 26). Frequently asked questions about animal drugs. U.S. Food and Drug Administration. https://www.fda.gov/animal-veterinary/safety-health/frequently-asked-questions-about-animal-drugs
FDA.gov. (2024, January 1). How can I tell if a drug is legally marketed for animals? U.S. Food and Drug Administration. https://www.fda.gov/animal-veterinary/unapproved-animal-drugs/how-can-i-tell-if-drug-legally-marketed-animals
Food Animal Residue Avoidance Databank. (2023). Prohibited and Restricted Drugs in Food Animals. http://www.farad.org/prohibited-and-restricted-drugs.html
Food Animal Residue Avoidance Databank. (2023). What is considered Extra-label Drug Use? http://www.farad.org/
Merck Animal Health. (n.d.). Banamine Label. Compendium of veterinary products. https://merckusa.cvpservice.com/product/basic/view/1047018
USAGov. (n.d.). Food and Drug Administration (FDA). https://www.usa.gov/agencies/food-and-drug-administration

